Like everything else in our universe, refrigerators have to obey a fundamental law of physics called the conservation of energy. The gist is that you can’t create energy out of nothing or make energy vanish into thin air: you can only ever convert energy into other forms. This has some very important implications for fridge users.
First, it busts the myth that you can cool your kitchen by leaving the refrigerator door open. Not true! As we’ve just seen, a refrigerator works by “sucking up” heat from the chiller cabinet with a cooling fluid, then pumping the fluid outside the cabinet, where it releases its heat. So if you remove a certain quantity of heat from inside your fridge, in theory, exactly the same amount reappears as heat around the back (in practice, you get slightly more heat given off because the motor is not perfectly efficient and it’s also giving off heat). Leave the door open and you’re simply moving heat energy from one part of your kitchen to the other.
The law of conservation of energy also explains why it takes so long to cool or freeze food in a refrigerator or a freezer. Food contains a lot of water, which is made from very lightweight molecules (hydrogen and oxygen are two of the lightest atoms). Even a small amount of water-based liquid (or food) contains a huge number of molecules, each of which takes energy to heat up or cool down. That’s why it takes a couple of minutes to boil even a cup or two of water: there are far more molecules to heat than if you were trying to boil something like a cup of molten iron or lead metal. The same applies to cooling: it takes energy and time to remove heat from watery liquids like fruit juice or food. That’s why freezing or cooling food takes so long. It’s not that your fridge or freezer is inefficient: it’s simply that you need to add or remove large amounts of energy to make watery things change their temperature by more than a few degrees.
Let’s try to put some rough figures to all this. The amount of energy it takes to change water’s temperature is called its specific heat capacity, and it’s 4200 joules per kilogram per degree celsius. It means you need to use 4200 joules of energy to heat or cool a kilogram of water by a single degree (or 8400 joules for two kilograms). So if you want to freeze a liter bottle of water (weighing 1kg) from a room temperature of 20°C to a freezer-like −20°C, you’ll need 4200 × 1kg × 40°C, or 168,000 joules. If your refrigerator’s freezing compartment can remove heat at a power of 100 watts (100 joules per second), that will take 1680 seconds or about half an hour.
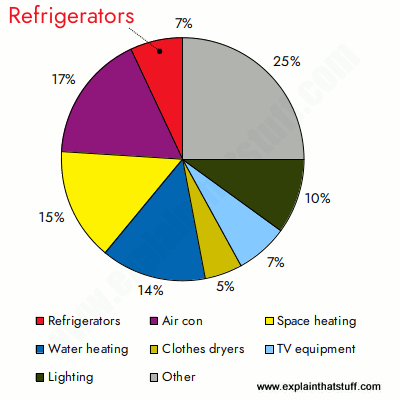
You can see that lots of energy is needed to cool watery foods. And that, in turn, explains why refrigerators use so much electricity. According to the US Energy Information Administration, fridges use about 7 percent of all domestic electricity (roughly the same as TVs and related appliances, and less than half as much as air conditioning, which uses a whopping 17 percent).
Chart: Home electricity consumption by end use: Refrigerators use 7 percent of domestic electricity—much less than air conditioners or heating systems. Main home refrigerators use about 77 percent of total refrigeration electricity, second fridges use another 18 percent, and further units account for the rest. Source: US Energy Information Administration,
Post time: Nov-02-2022